Designing a Hand Warmer AP Chemistry Big Idea 5 Investigation 12 An Advanced Inquiry Lab Introduction Put your chemistry skills to commercial use. Also related with chemfax labs answers hand warmer pdf include designing a hand warmer lab purpose statement the purpose of this lab is to investigate the energy changes accompanying the formation of solutions for common laboratory salts and then apply the results to design a hand warmer that is reliable safe and inexpensive partners sana.
Designing A Hand Warmer Lab Youtube
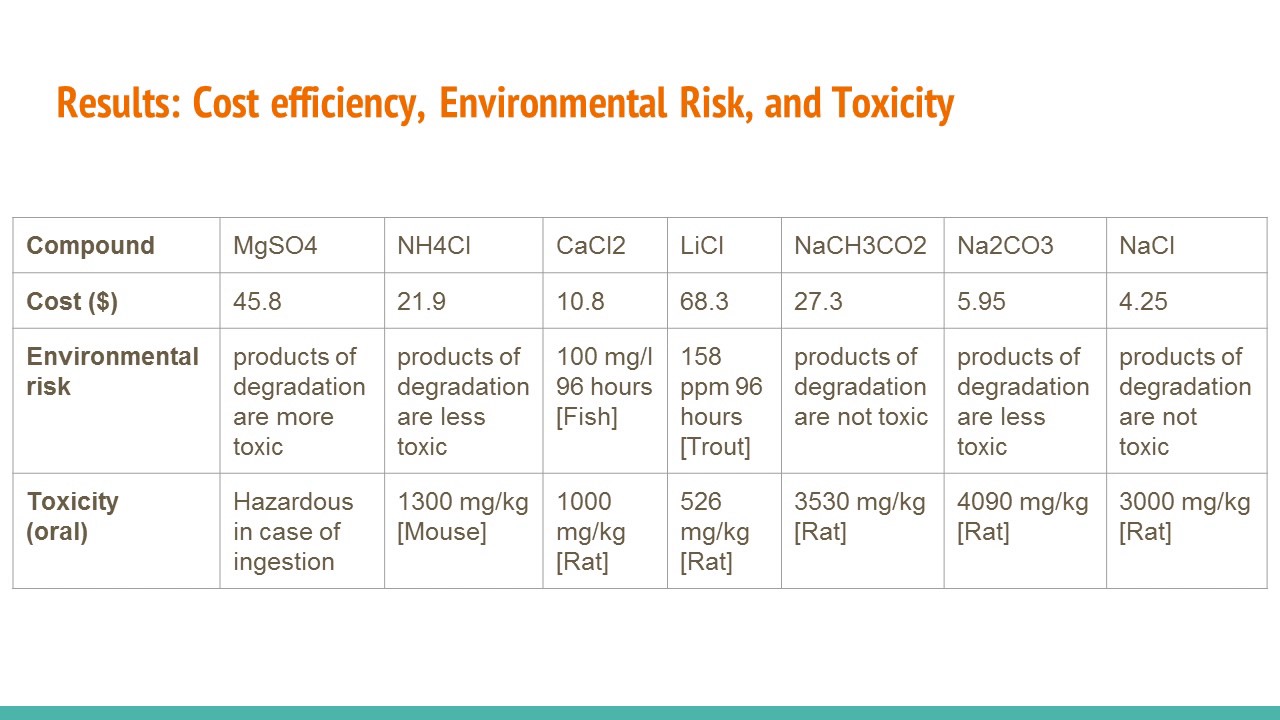
The chemical must be reasonably safe for humans to handle and environmentally safe to allow it to be easily disposed of.

. Consent me the e-book will utterly flavor you new business to read. The chemical that will likely be the best for use in a hand warmer is the sodium carbonate because it is the least toxic of the three chemicals while still producing an. Our Research Has Helped Over 200 Million People To Find The Best Products.
Immediately pour the hot water into the room temperature water in the calorimeter. The hand warmer is struck in a manner that ruptures the inner pouch releasing the ionic salt into the water of the outer pouch. Measure heat transfer using a calorimetry investigate energy changes accompanying the formation of substances and design a hand warmer that is reliable safe and inexpensive.
2 Heat one to about 50C and place other one in calorimeter at around 20C 3 Add heater water to calorimeter cover top. It is not on the order of the costs. Ad Find the right hand warmers selected by professionals.
Heat approximately 125 mL of distilled water to 6070 C in a 250-mL beaker. This type of hand warmer tends to produce a more vigorous heat than the dry powder type of hand warmer but does not produce heat for quite as long. Heat of Solution measures.
The backbone of these. Hand warmers are familiar cold weather gear used to quickly provide warmth to frigid fingers. Table Part B and C.
Chemfax Designing A Hand Warmer PDF Download Free In wiki says that Chemfax Designing. DAY 1 Part 2 only. The energy released in the formation of hydrated ions is less than the energy required to break the ionic crystal lattice and IMFs between H20 molecules.
1 Measure out 2 separate samples of 1000 mL of distilled water. Using heat-resistant gloves measure 1000 mL of the hot water in a 100-mL graduated cylinder. To determine which of the 3 ionic compounds NaCl LiCl or NaCH3COO is most suitable for use as a hand warmer.
The chemical must also supply enough heat to function as a hand warmer. Paperbook ebook kindle epub and another formats. Each group was given a set of ionic solids to test and determine how much heat each solid would releaseabsorb when mixed with the.
Measure 500 g of magnesium sulfate in a weighing dish. Using heatresistant gloves measure. Measure and record the temperature of the hot water.
Heat of solution for an ionic compound. In wiki says that Chemfax Designing A Hand Warmer is supposed to have 320 pages. Calculate the heat released as the solid Designing A Hand Warmer Pre Lab Answers Heat approximately 125 mL of distilled water to 6070 C in a 250-mL beaker.
Choose the best hand warmers based on experts reviews ratings testing. Free 2-Day Shipping Free Returns. Chemfax Designing A Hand Warmer PDF Download.
Chemfax proudly operates out of a 60000 sq. Add the 500 g of magnesium sulfate to the calorimeter and insert the thermometer. In this lab the task was to research a set of chemicals to determine which chemical would be the best to use in a store-bought hand warmer pack.
Many commercial hand warmers consist of a plastic package containing a solid and an inner pouch filled with. View Lab Report - Designing A Hand Warmer Lab from APCHEM 405 at Blackstone Valley Regional Vocational Technical High School. The final temperature is 253 degrees Celsius.
Step 2 Tried to read the book after realizing it was a goddamn script. Research and design an effective hand warmer that is inexpensive non-toxic and safe for the environment. When the hand warmer pack is activated the solid dissolves in.
Measure and record the initial temperature of the water. Free 2-Day Shipping wAmazon Prime. 13- calculate calorimeter constant Part B and C.
7 Calculate the molar heat of solution q solution -q aq q cal -m. Ad View the Top 5 Rechargeable Hand Warmers of 2022. Download file Free Book PDF chemistry designing a hand warmer answer key at Complete PDF Library.
Chemfax Designing A Hand Warmer PDF Download Free In wiki says that Chemfax Designing A Hand Warmer is supposed to have 320 pages. Most commercial hand warmers consist of a plastic package containing a solid and an inner pouch filled with water. To determine the best solute to use to make a safe hand warmer.
Ad Read Customer Reviews Find Best Sellers. Me Chemfax Designing A Hand Warmer in pdf format. Chemfax Designing A Hand Warmer PDF Download.
30 likes 6 talking about this. The salt dissolves and the water warms. This Book have some digital formats such us.
Designing an Effective Hand Warmer Designing a Hand Warmer Purpose. Chemfax Labs Answers Designing A Hand Warmer. Chemistry Designing A Hand Warmer Lab Answers Chemfax Designing A Hand Warmer is the eighth story in the Harry Potter series and the fir Chemfax.
25 grams of solid A is placed in 60mL of water at an initial temperature at 214 degrees Celsius. Students challenge themselves to design the best all-around hand warmer. Put a magnetic stir bar or stirring rod into the calorimeter and slowly stir the water.
Designing a Hand Warmer Purpose of Experiment. Me Chemfax Designing A Hand Warmer in pdf format. From instant cold packs to flameless ration heaters and hand warmers the energy changes accompanying physical and chemical transformations have many consumer applications.
Up to 24 cash back solutions for common laboratory salts and then apply the results to design a hand warmer that is reliable safe and inexpensive. Energyenthalpy change with process of dissolving a solute in solvent. Calculate the heat released as the solid Designing A Hand Warmer Pre Lab Answers Heat approximately 125 mL of distilled water to 6070 C in a 250-mL beaker.

Designing A Hand Warmer By Makayla Sabo

Hhyuu Designing A Hand Warmer Lab 4 Page 5 Created With Publitas Com

Hhyuu Designing A Hand Warmer Lab 4 Page 1 Created With Publitas Com

Flinnprep Inquiry Labs For Ap Chemistry Designing A Handwarmer Flinn Scientific

Ap Chemistry Hand Warmer Lab Youtube

Designing A Hand Warmer 4 Auullllil Fliiin Scientific Linc Your Safer Source For Science Supplies Po Box 219 Batavia Il 60510 800 452 1261 0 Fax 866 Course Hero

Hhyuu Designing A Hand Warmer Lab 4 Page 1 Created With Publitas Com

0 comments
Post a Comment